TactiCath

TactiCath™ Contact Force Ablation Catheter, Sensor Enabled™ is an innovative solution for the treatment of atrial fibrillation featuring advanced handle-shaft technology and full integration with Abbott’s EnSite Precision™ Cardiac Mapping System.
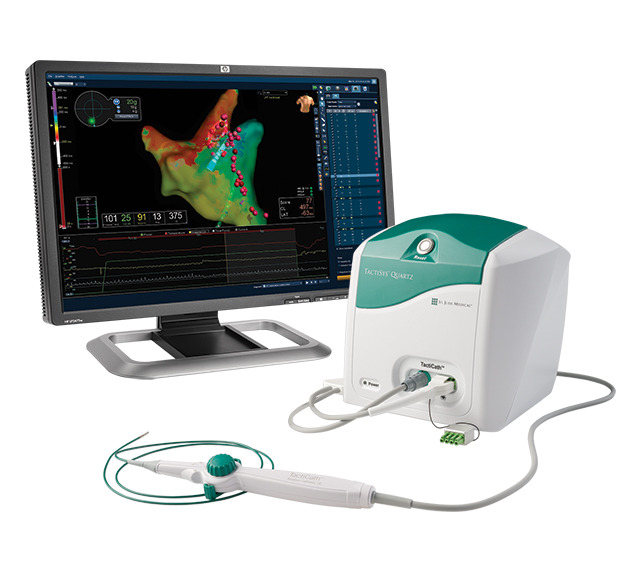
TactiCath Contact Force Ablation Catheter, Sensor Enabled
Accurate
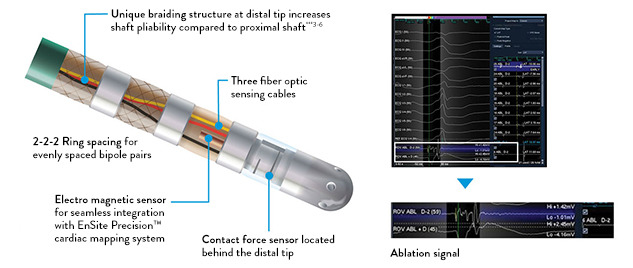
At the heart of the TactiCath Contact Force Ablation Catheter, Sensor Enabled is light interferometry technology. During bench testing, the TactiCath Contact Force Ablation Catheter, Sensor Enabled was highly accurate, with a mean accuracy of 0.3 grams. 1
Effortless handling 2*
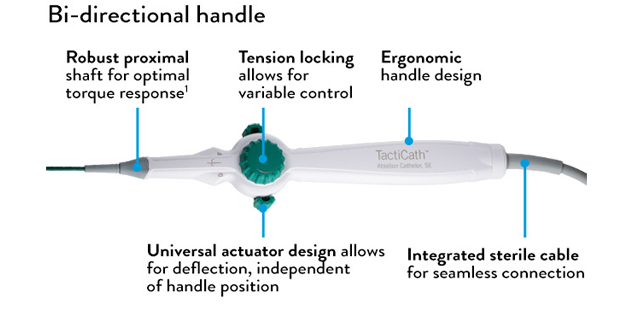
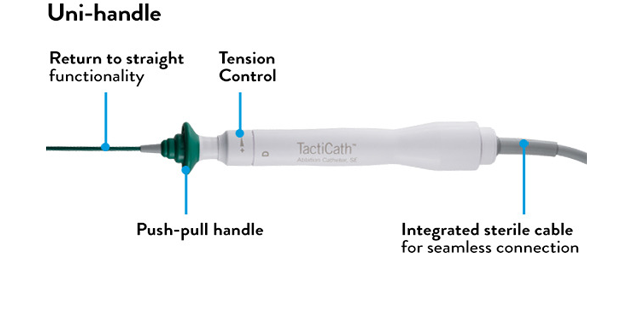
- Advanced handle-shaft combination offers maneuverability, along with comfort and ease of use 2
- A variety of curve sizes accommodates size differences in patient anatomy
Integrated 3,4
- Get novel insight into mapping and lesion marking through AutoMark feature advanced technology.
- Increase procedural consistency through automated guidance of lesion marking via the AutoMark feature. 3
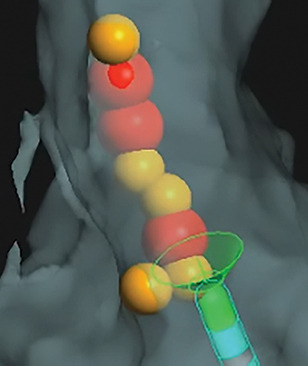 Cardiac mapping image with the Metrics integration display of the TactiCath Contact Force Ablation Catheter, SE." width="" height="" />
Cardiac mapping image with the Metrics integration display of the TactiCath Contact Force Ablation Catheter, SE." width="" height="" />
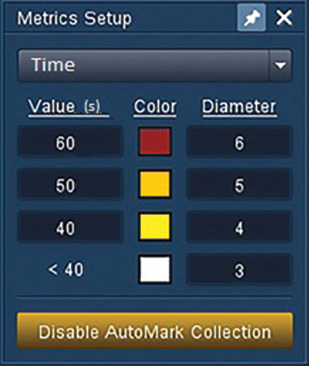
- Verify ablation catheter stability and AutoMark placement with the AutoTrack feature, which automatically records the precise location of the tip during radio frequency energy application. 2
- Review and identify any potential gaps by viewing specific lesions from the display list.
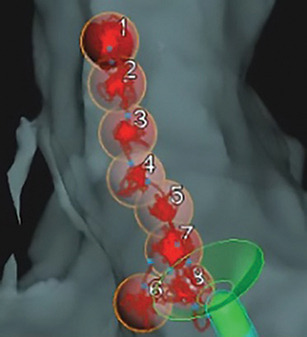
- Optimize your workflow with customizable CF display.
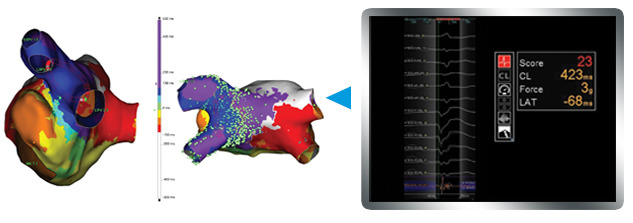
- Map with precision via EnSite™ AutoMap module technology — designate the system to collect mapping points only if the CF is within a specified range. 3,4
- Uniquely integrate magnetic and impedance data.
- Adapt to changing needs — introduce the TactiCath Contact Force Ablation Catheter, Sensor Enabled at any point during the procedure. 3,4



*Effortless handling is based on how physicians scored catheter handling characteristics during an initial market release.
References
- Abbott. Data on File. Report 90430651
- Abbott. Data on File. Report 90349982.
- Abbott. Data on File. Report 90214738.
- Abbott. Data on File. Report 90253949.
POLICIES & ADVISORIES
- Advertising Preferences
- Consumer Health Data Privacy Policy
- Contact Us
- Customer Service
- Policies
- Privacy Policy
- Product Advisories
- Terms and Conditions
HEALTHCARE PROFESSIONALS
- Disease Management
- Products
- Education & Training
- Reimbursement
- Manuals & Technical Resources
- MRI Safety
- Investigator Sponsored Studies
- Product & Charitable Donations
- Product Performance Reports
PATIENTS & CAREGIVERS
- Treatments & Therapies
- Manage Your ID Card
- Traveling with Your Device
- Get Support
Stay Connected
CAUTION: These products are intended for use by or under the direction of a physician. Prior to use, reference the Instructions for Use, inside the product carton (when available) or online for more detailed information on Indications, Contraindications, Warnings, Precautions and Adverse Events.
Illustrations are artist's representations only and should not be considered as engineering drawings or photographs.
Unless otherwise specified, all product names appearing in this Internet site are trademarks owned by or licensed to Abbott, its subsidiaries or affiliates. No use of any Abbott trademark, trade name, or trade dress in this site may be made without the prior written authorization of Abbott, except to identify the product or services of the company. ™ Indicates a trademark of the Abbott group of companies. ‡ Indicates a third party trademark, which is property of its respective owner.
© 2024 Abbott. All Rights Reserved.







 Cardiac mapping image with the Metrics integration display of the TactiCath Contact Force Ablation Catheter, SE." width="" height="" />
Cardiac mapping image with the Metrics integration display of the TactiCath Contact Force Ablation Catheter, SE." width="" height="" />


![]()
![]()
![]()